This is the last article you can read this month
You can read more article this month
You can read more articles this month
Sorry your limit is up for this month
Reset on:
Please help support the Morning Star by subscribing here
IN THE coming weeks and months, the National Health Service will be tested to its limits, striving to save as many patients as possible who are seriously ill due to the coronavirus.
The surges in intensive-care patient numbers will cause major disruption to the routine work of the NHS, which includes crucial life-saving procedures.
One such procedure that has been particularly affected by international travel disruption has been stem-cell donation, for the treatment of conditions such as blood cancer.
Once the donation has been made, the stem cells need to be transplanted into the recipient within 72 hours.
Our immune system uses special structures on the surfaces of cells called antigens to recognise cells that belong to our own body (“self”).
Materials in the body without the correct genetic passcode are labelled as “non-self” and need to be destroyed since they may be bacteria, viruses, and parasites that can harm us.
To do so, our immune system makes targeted molecules called antibodies that bind to specific antigens and tag them for attack by immune cells.
When we are exposed to something for the first time, we lack the right antibodies to label the invasive virus for destruction.
We can acquire these antibodies through infection as we fight off the illness, or through vaccination, which introduces an inert form into our blood.
Over a period of time our bodies learn to identify and destroy the “non-self” material effectively and we stop reproducing it and passing it to others.
This is the opposite of what is desirable in a stem-cell transplant. Here, the stem cells must have matching human leukocyte antigens (HLA) to prevent the new host body from rejecting them as “non-self” and attacking the life-saving stem cells with the body’s own immune system.
The genes that make up cell antigens are very diverse. In general, this is very good for humanity because it means that each new generation of humans has new mutations in its immune system, as you receive a fresh mix of genes from your parents.
These immune mutations can be beneficial, in which case the humans with them will be less likely to succumb to disease and more likely to live on to pass on the mutation to their offspring.
The recombination of genes in each generation makes it possible for the population as a whole to stay one step ahead of our pathogenic enemies.
The extreme variation in cell antigens is, however, bad news when it comes to searching for a match between stem-cell donors and recipients.
Your most likely option for a match comes from a sibling. Since each parent has two sets of genetic code for cell antigens that you can inherit, you have a roughly one-in-four chance of sharing your antigen genes with your sibling.
Testing to see whether your antigen genes match with a stranger involves checking for a match across 10 different locations in the genes (referred to as “markers”) that signify a high likelihood of compatibility; some of these gene sequences have hundreds of varieties, so it becomes extremely unlikely that you will fully match with someone.
This is where bone-marrow databases come in. Testing your markers against thousands of individuals increases the probability that you will find a match. The more people that are signed up to the database, the more matches will be made.
For people requiring a transplant it is vital to find a match. Stem-cell transplants are required when the host’s bone marrow is no longer capable of producing stem cells or when it has been destroyed as an unfortunate side effect of treatment for blood cancer.
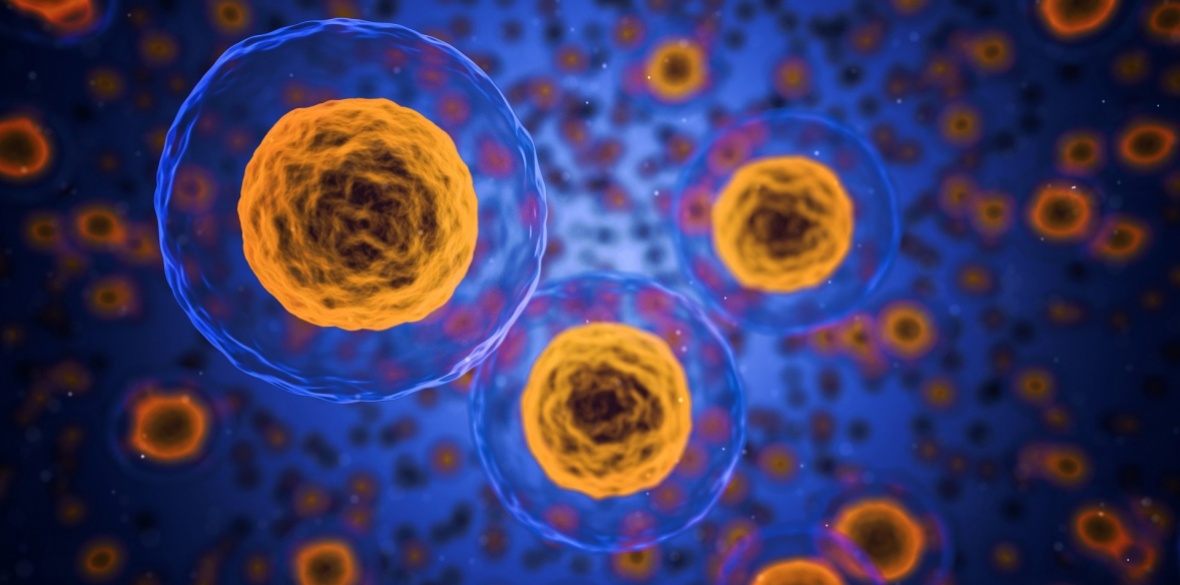
Bone marrow is the main site of the process known as haematopoiesis: the production of new blood cells. Healthy bone marrow is full of stem cells that produce new blood cells.
Bone-marrow stem cells transform into a wide variety of cells in the blood. These include red blood cells that carry oxygen around the body, megakaryocytes that produce platelets to help blood clot, and neutrophils that consume “non-self” cells such as bacteria.
Crucially, bone-marrow stem cells reproduce themselves to keep their population at a constant level.
If the stem-cell population crashes, then so will the population of neutrophils (and other blood cells) in the blood.
The idea of a stem-cell transplant is to replace this population in people where it has been depleted.
An example of a disease that can be treated with a stem-cell transplant is leukaemia: a type of cancer that begins in the bone marrow and produces mutated blood cells.
The collection of different cells produced by bone-marrow stem cells make up the whole of what we call the immune system.
Often the course of treatment requires chemotherapy or radiotherapy, both of which also further damage the immune system and bone marrow.
A weakened immune may not be able to respond with full strength, putting people in this condition at particular risk from new infections, including coronaviruses.
A stem-cell transplant aims to fix this by giving them a new immune system, produced by fully functioning stem cells.
On the donor’s part, the transplant requires four days of injections beforehand that encourage the release of stem cells from the bone marrow into the bloodstream.
The donor then undergoes dialysis, and a centrifuge is used to separate the relevant stem cells from the rest of the cells in the blood, which is returned to the body.
When the stem cells are transfused into their new body, some of them will have already started transforming into all sorts of new blood cells and will immediately function as a temporary immune system boost.
However, the real goal of the transplant is for some of the stem cells to enter the recipient’s bone marrow and establish themselves there.
It will take from months to years for the transplanted stem cells to produce enough new stem cells and other blood cells to constitute a fully functioning immune system.
Since the recipient’s original immune system was completely destroyed before the transplant — which also helps to prevent their body rejecting the new transplant — their new immune system will be rebuilt by a regenerating lineage of stem cells that are entirely derived from the donor.
Stem-cell donation is a marvel of modern medicine, treating conditions that were incurable under a century ago.
The Covid-19 pandemic does not mean other problems have gone away. Even during these uncertain times, the search for stem-cell donors continues.